From holding up the roof above your head, to getting you between A and B, concrete is used in a myriad of ways that make our lives what they are.
Concrete, a mix of tri-calcium silicates (otherwise known as cement), sand, aggregates (as well as few other plasticity modifiers, setting retardants, etc.) and most importantly, water, is a compound that has been studied by people (not unlike us at Microanalysis) since before the Romans learned how to take a bath.
Once wetted, the hydration products start to form and concrete begins to set – hey-presto, your ornate mosaic tiles are hopefully forever fixed to your steam room floor! What you actually end up with is hopefully a solid mixture of fine air voids, capillary pores, hydration products, unreacted cement and sand/aggregates. But, get the mix wrong and you’ll have more than Caesar’s face sticking to your feet.
Non-compliance these days can see investigations into water:cement and sand:cement ratios as well as aggregate types (alkali-silicate reaction) and non-heterogeneity of mix. Traditional methods employ lengthy physio-chemical techniques such as BS1881:124:1998 but have been suspected of large inaccuracies for many years (A.M.Neville (2003), “How closely can we determine the water-cement ration of hardened concrete”, Mat. Struct. 36, 311-318.).
Now the clever people at Imperial College London have published a paper (http://www3.imperial.ac.uk/concretedurability/researchprojects/determiningwatercementratio) outlining a relatively simple method using scanning electron microscopy for concrete petrography.
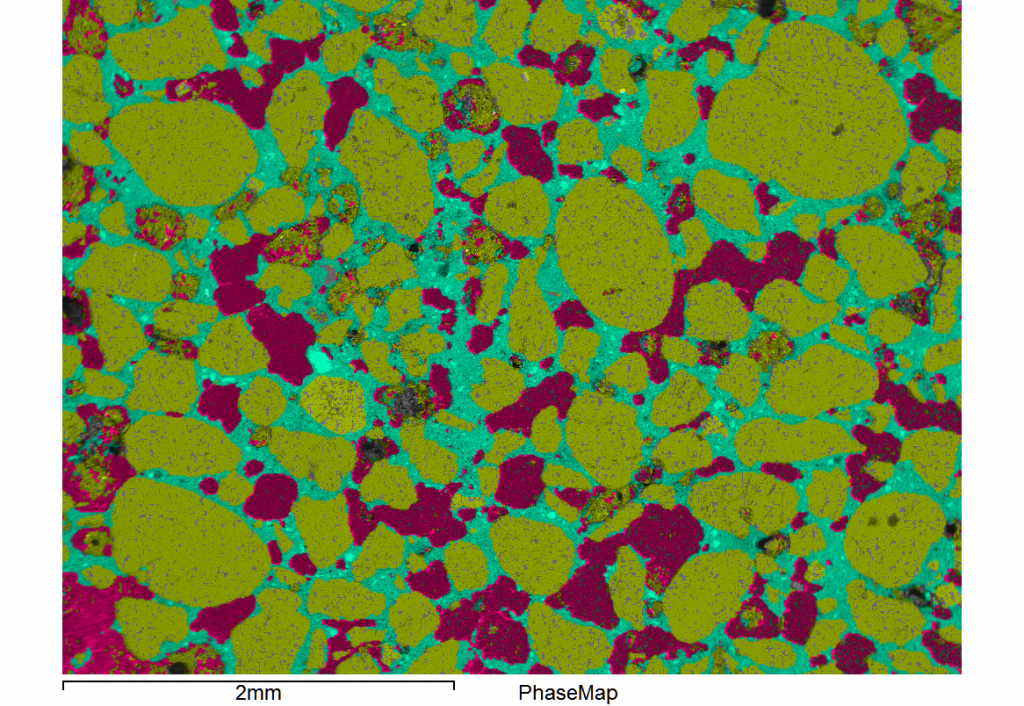
Apart from obtaining colourful images, the method allows you to determine the volume fraction of reacted /unreacted cement, capillary pores and hydration products. So, a quick thin-section, carbon coat and 15 minutes worth of x-ray mapping and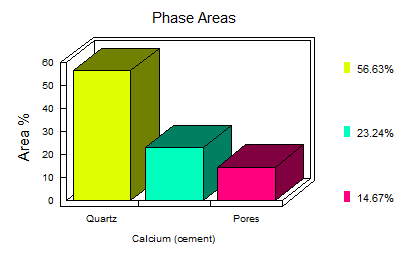 the images below can be interpreted to report both the
the images below can be interpreted to report both the
water:cement and sand:cement ratios as well as an in-depth interpretation of swelling aggregates (ASR), ettringite formation (sulphate presence), carbonation, chloride penetration and calcium dissolution/leaching.